IDC Product Design in the UK Plastics with leading manufacturer Shaily Engineering to discuss the development of a common drug injection pen platform. Once a pharmaceutical company customer wants a three-dose injection pen, they will quickly follow up.
Such platforms are often highly protected by patents, which makes the development of new devices challenging. The UK IDC team found that patents on several structures of existing drug delivery devices have expired, which opens the door for us to develop new platforms with independent intellectual property rights.
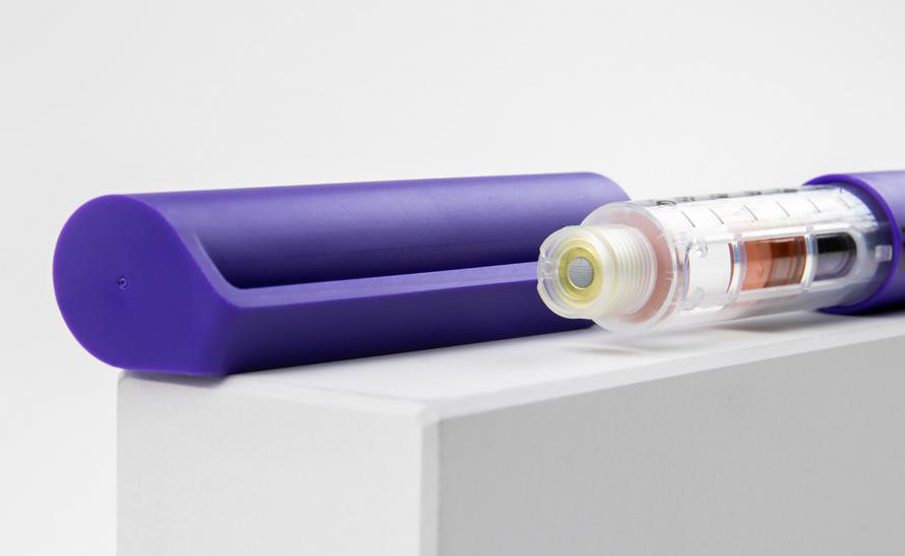
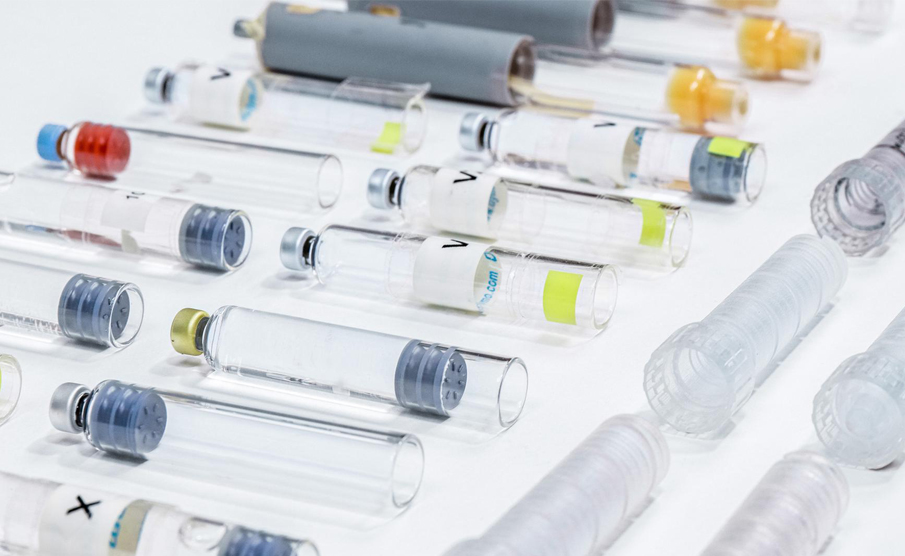
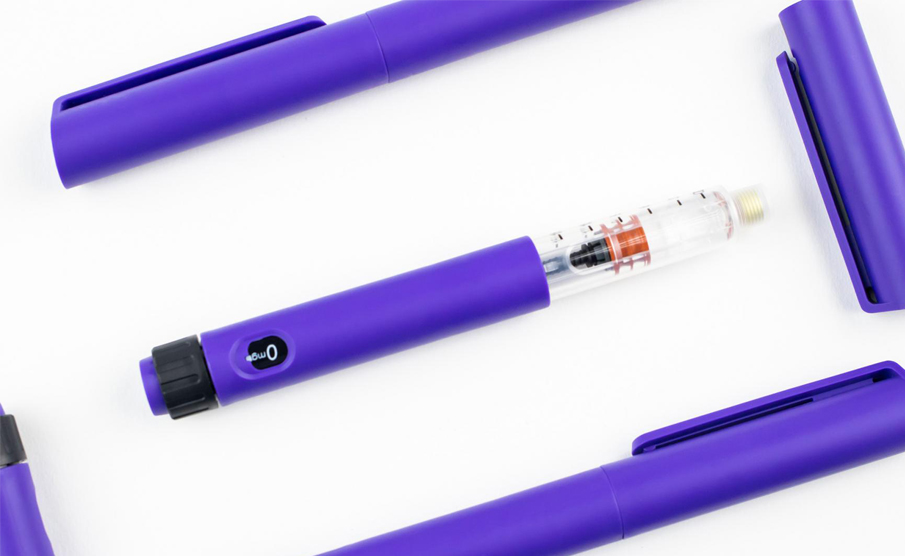
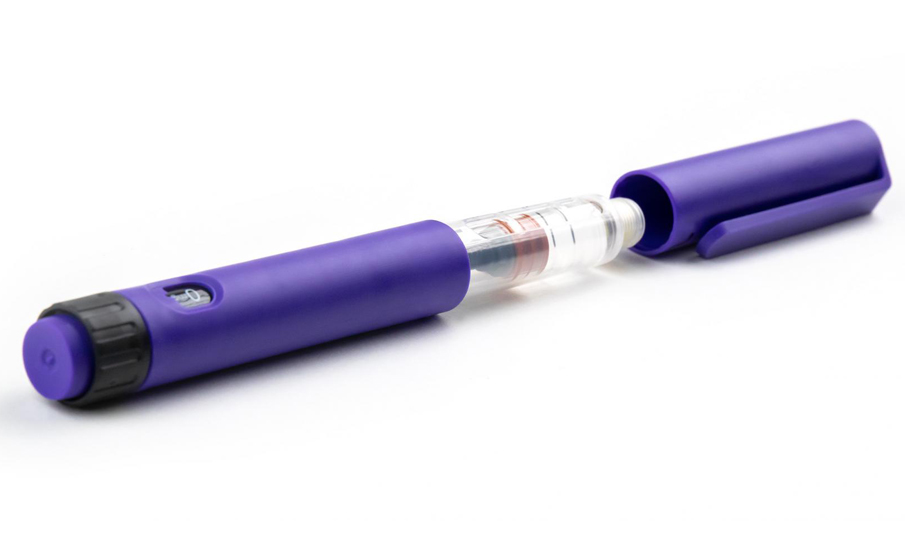
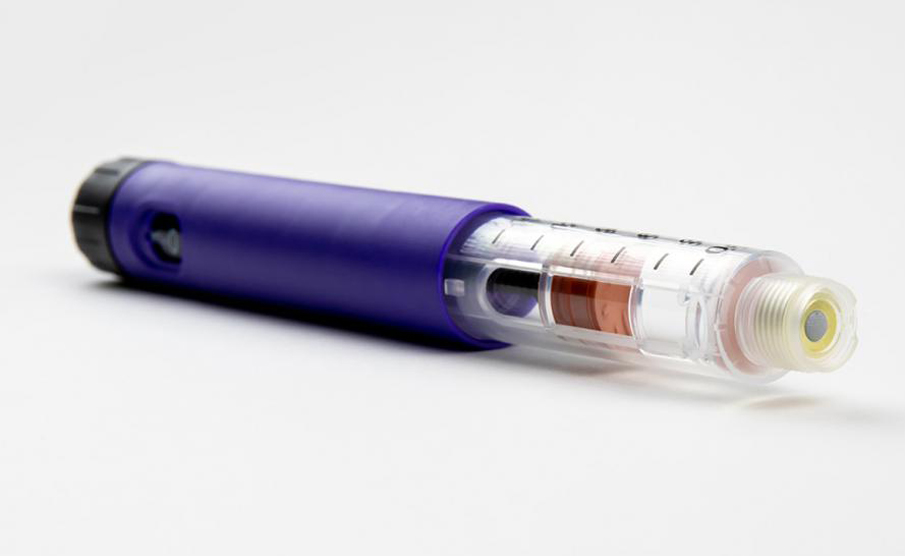
In collaboration with Shaily and its manufacturing experts, they briefed IDC UK on the development of high quality disposable pen platforms for single-dose, three-dose and 60-unit insulin variants (single-and reusable variants) for administration, such as abalopeptide, liraglutide or insulin glargine, which all use the same core structure. The device will be manufactured at Shaily's world-class facility, and its sub-components will be finally assembled with pre-filled cartridges at the pharmaceutical company.
IDC has rich experience in the design and development of drug injection pens.
The knowledge the team has is particularly valuable, it allows the UK IDC team to have a comprehensive and clear understanding of the product and its development process, without the need for a long period of time. The existing syringe platform still has multiple patents, and IDC engineers in the UK have worked hard to avoid infringement while designing new internal features-down to the way the external buttons connect to the inside of the device.
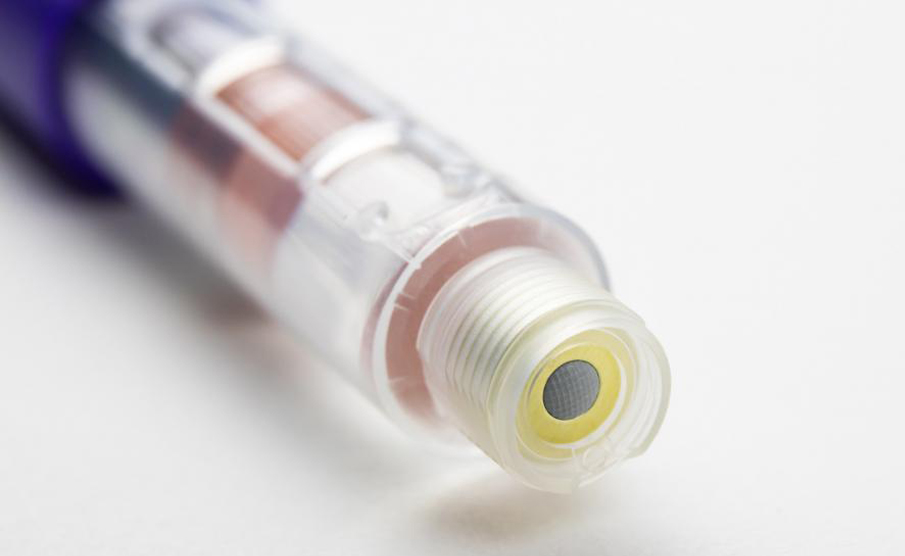
The safety and accuracy of the product is the top priority. The IDC team in the UK specially designed the "final dose locking" structure and the patented assembly method to prevent the user from taking the residual medicine in the medication cartridge again after the final dose delivery is completed.
Protean materials are plastic, using high-performance biocompatible plastics from DuPont, Celanese and Nordic Chemicals. The design team IDC UK carefully tests and selects plastics to meet excellent dimensional stability, can meet strict equipment tolerance ranges, and works closely with pharmaceutical companies to develop cartridges suitable for equipment, and provides advice on materials, design and assembly processes.
We use injection molded parts to make prototypes and test, to ensure realistic material force and motion accuracy, to ensure the accuracy of the dose performance. In the early stages of development, the use of SLA technology that enables rapid development can help product visualization and general functionality. Our project manager oversaw the entire mold manufacturing process in Taiwan and worked closely with Shaily and pharmaceutical companies to bring the design into production.
As the plan is first launched in the United States, IDC in the UK provides documentation for the full development process for FDA review and certification, and directly interfaces with public trust testing institutions to supervise 11608 design verification testing. Maintaining good communication throughout the project means that we can deliver excellent designs within the time frame that our customers are satisfied. Currently, single-dose and 60-unit dosing devices are also in production along with reusable insulin variants.
"We have selected IDC UK as our partner for many of our current drug delivery device projects, each time they have exceeded our expectations and provided excellent design and development services for complex technical problems in a short period of time. Their ability to develop new intellectual property for our business in a challenging market is extremely important to us."
--Amit Sanghvi Shaily
www.idcdesigncn.com


本作品版权归 英国IDC产品设计 所有,禁止匿名转载及个人使用,任何商业用途均需联系原作者。

新用户?创建账号
登录 重置密码

请输入电子邮件以重置密码。
Oh ho
it is very convenient to carry
Not bad
very easy to use