When doing market research on medical product design, it is necessary to improve the expected use, performance, efficacy, safety and reliability of medical products according to the performance, function, materials used and risk management requirements of medical products. The production environment also has certain requirements, service life and other aspects. After detailed review, confirmation and approval, corresponding documents are formed.
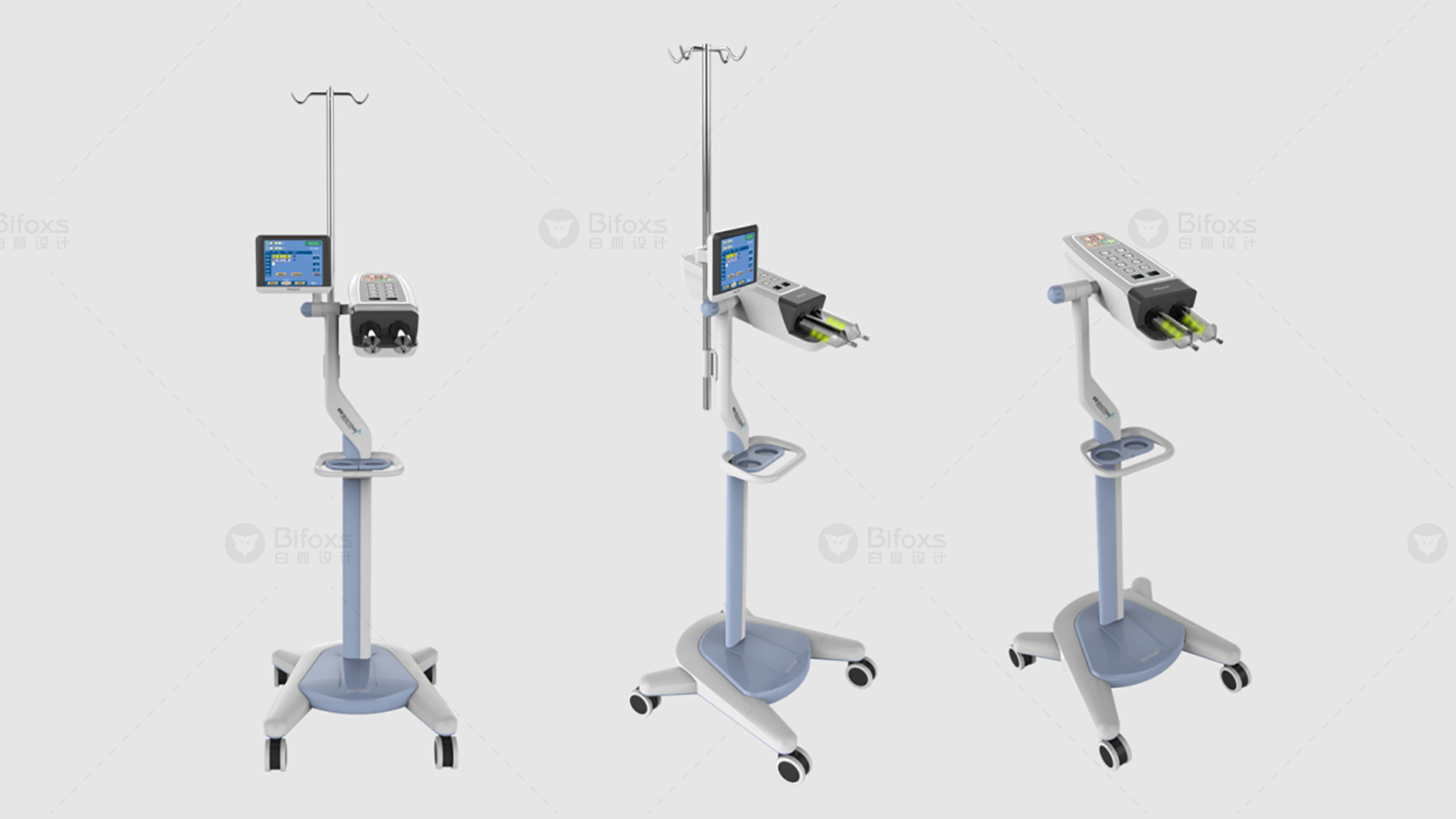
Digital enterprises in the medical device industry represent the overall approach to product lifecycle management across the conception, realization and utilization stages of the device product lifecycle. Our customers weave digital threads in a flexible and precise way throughout the product life cycle, thereby leveraging the power of digitization through the seamless connection of digital twins.
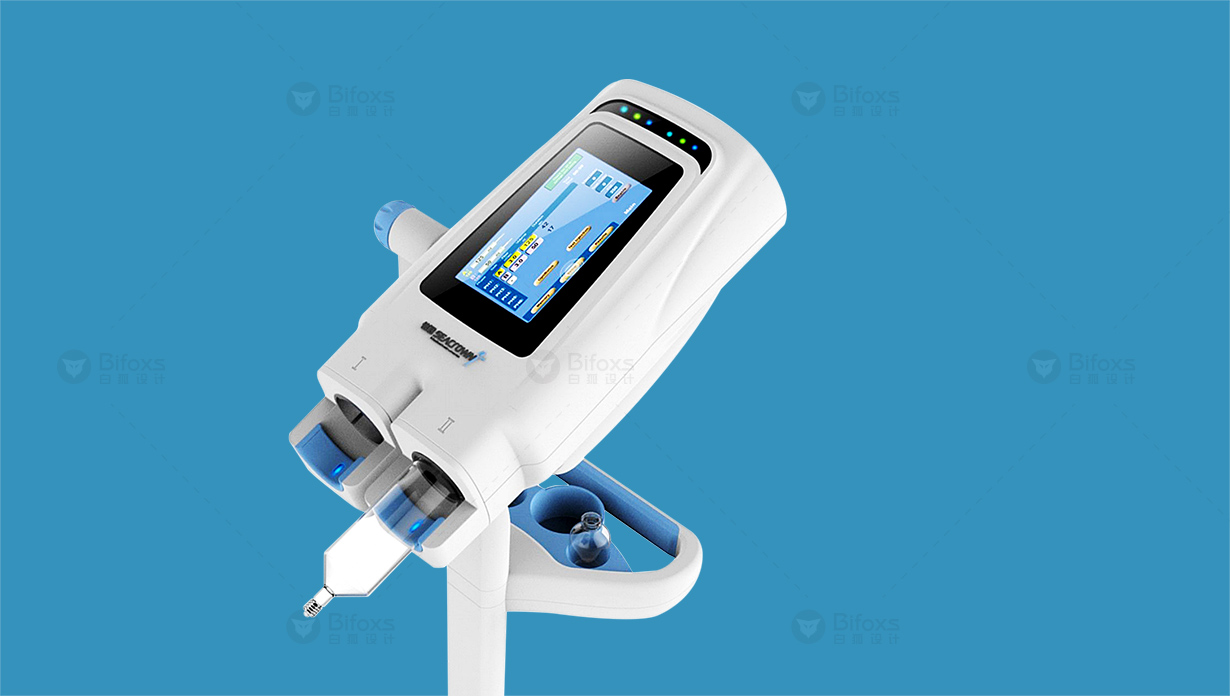
Provide us with powerful and unique functions, especially the use of digital twins at every stage of the equipment product life cycle. This not only represents the digital twin equipment in the conception and utilization stage, but also represents the digital twins in the factory and the production and processing process at the present stage.
Digital twins provide power for digital simulation, enabling our customers to predict, optimize and realize the engineering performance and quality of products and production and processing businesses.
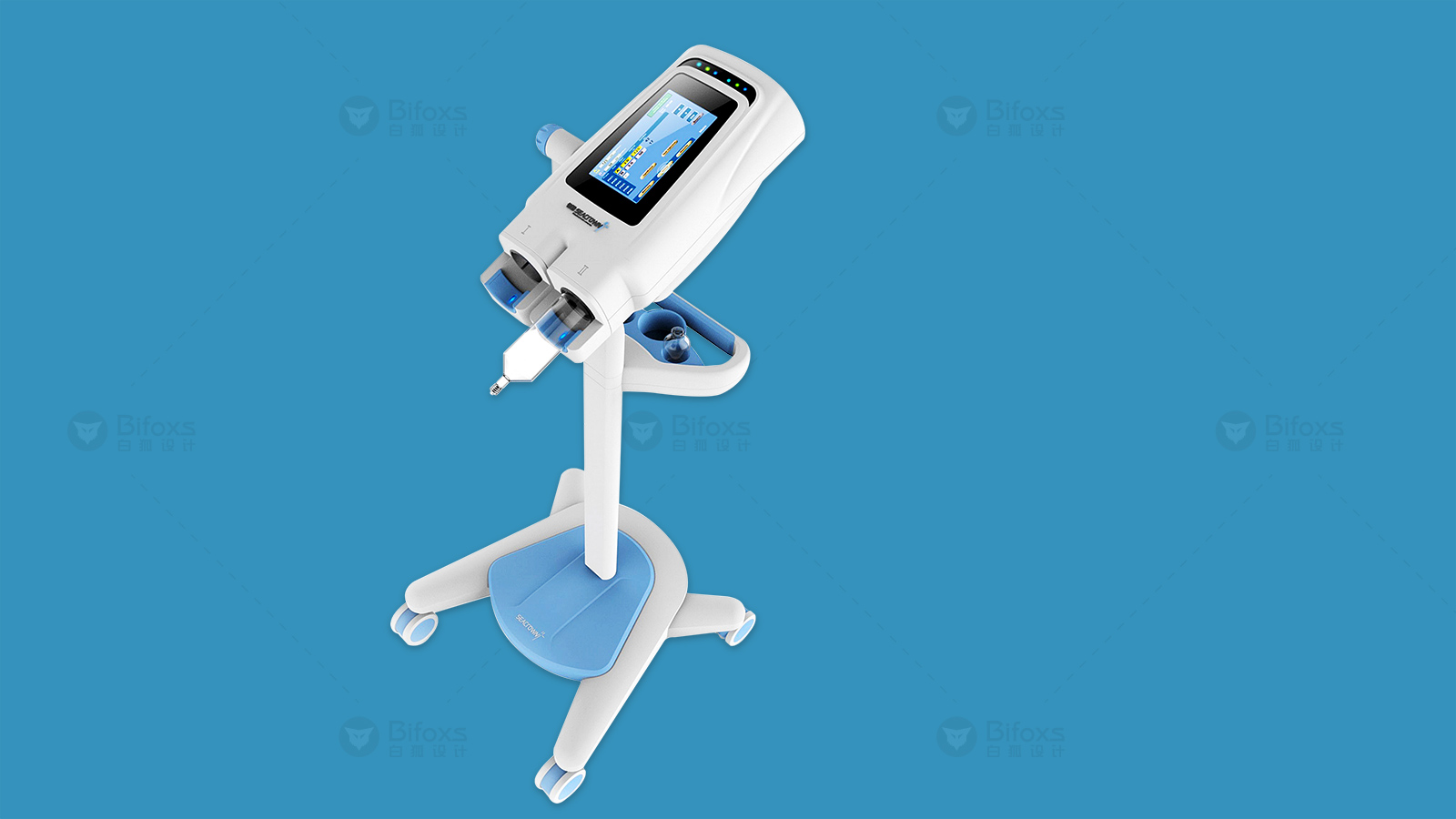
In addition, digital threads are woven into design, supplier and quality control to provide traceability across functional boundaries, engineering disciplines and product life cycle stages. These digital threads, combined with big data analytics, can generate insightful reports and identify trends, resulting in better and more effective medical product designs.
本作品版权归 白狐设计 所有,禁止匿名转载及个人使用,任何商业用途均需联系原作者。

新用户?创建账号
登录 重置密码

请输入电子邮件以重置密码。
留言板 (0)
评论为空